At SignifyQ, we understand that Quality is more than a regulatory requirement — it’s the foundation of trust, reputation, and business sustainability. Our Quality Management System (QMS) Solutions empower pharmaceutical, biotech, and medical device organizations to establish, maintain, and continuously enhance quality frameworks that meet global standards while optimizing performance.
Our QMS Service Spectrum
Quality Risk Management
Our experts apply ICH Q9 principles to establish proactive risk identification, evaluation, control, and review processes across your product lifecycle.
Deliverables:
QMS Design, Development & Implementation
We develop tailored QMS frameworks aligned with FDA, EMA, MHRA, WHO, and ISO 9001/13485 standards. From policy drafting to SOP structuring and electronic QMS implementation, we ensure that every process integrates quality into its core.
It includes:
Deviation, Change Control & CAPA Systems
We design robust systems to track and resolve non-conformities through structured deviation handling and CAPA methodologies, ensuring root causes are addressed effectively.
Our Approach:
GxP Compliance Audits & Inspection Readiness
We conduct GxP (GMP, GDP, GCP, GLP, and GVP) audits, helping clients maintain continuous compliance and readiness for FDA, EMA, or local authority inspections.
Key Focus Areas:
Supplier Quality & Vendor Qualification
End-to-end supplier lifecycle management, from selection to qualification and ongoing performance monitoring.
We Handle:

QMS Design, Development & Implementation
We develop tailored QMS frameworks aligned with FDA, EMA, MHRA, WHO, and ISO 9001/13485 standards. From policy drafting to SOP structuring and electronic QMS implementation, we ensure that every process integrates quality into its core.
It includes:

Quality Risk Management
Our experts apply ICH Q9 principles to establish proactive risk identification, evaluation, control, and review processes across your product lifecycle.
Deliverables:


GxP Compliance Audits & Inspection Readiness
We conduct GxP (GMP, GDP, GCP, GLP, and GVP) audits, helping clients maintain continuous compliance and readiness for FDA, EMA, or local authority inspections.
Key Focus Areas:

Deviation, Change Control & CAPA Systems
We design robust systems to track and resolve non-conformities through structured deviation handling and CAPA methodologies, ensuring root causes are addressed effectively.
Our Approach:


Supplier Quality & Vendor Qualification
End-to-end supplier lifecycle management, from selection to qualification and ongoing performance monitoring.
We Handle:
Our QMS Methodology
Our QMS consulting follows a Plan–Implement–Validate–Sustain (PIVS) model:
Key Benefits
Regulatory Consulting
SignifyQ is your global partner in Regulatory Strategy, Submission, and Compliance
Comprehensive Regulatory Services
Dossier Preparation & Submission Management
We manage complete submission lifecycles for drug, biologic, and device applications — from authoring to publishing and lifecycle maintenance.
Key Deliverables:
Regulatory Strategy & Intelligence
We develop proactive regulatory roadmaps customized to your product type, target market, and clinical development phase.
Our Strategic Focus:
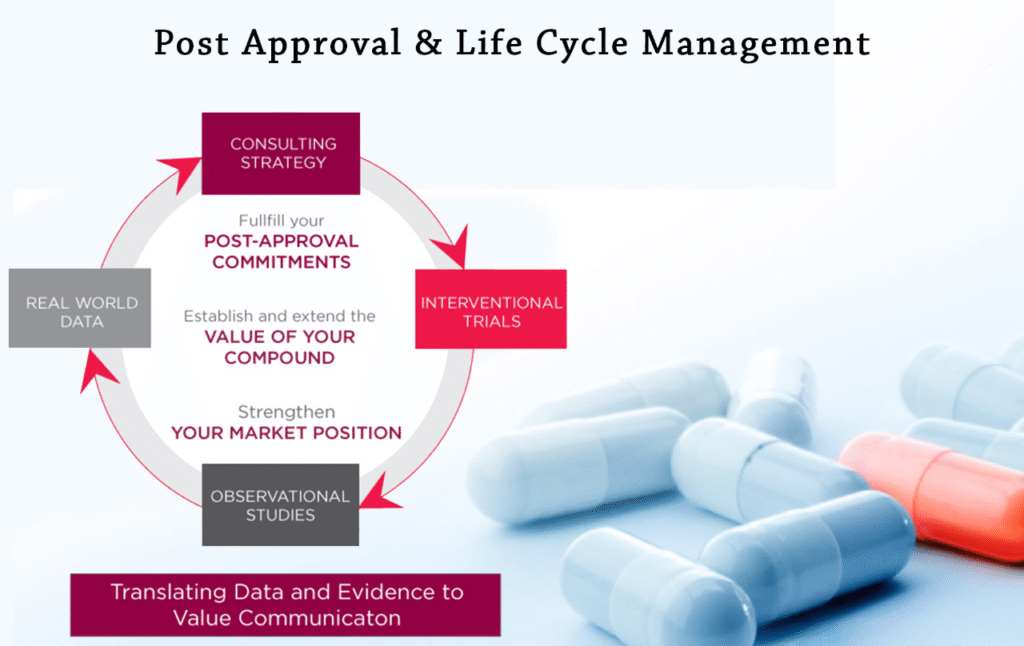
Post-Approval Lifecycle Management
From variations to renewals and labelling updates, we ensure your products remain compliant throughout their market lifecycle.
Our Support Covers:
Health Authority Interactions
Our experts prepare and represent clients during Health Authority communications, meetings, and inspections — providing confidence through experience.
Includes:
Digital & Data-Driven Regulatory Operations
We implement modern digital tools and AI-driven data analytics for smarter, faster, and more accurate submission processes.
Digital Initiatives:

Regulatory Strategy & Intelligence
Our Strategic Focus:
Dossier Preparation & Submission Management
Key Deliverables:


Health Authority Interactions
Includes:

Post-Approval Lifecycle Management
Our Support Covers:


Digital & Data-Driven Regulatory Operations
Digital Initiatives:
Our Regulatory Philosophy
We integrate science, compliance, and strategy to deliver end-to-end regulatory solutions — ensuring your submission not only meets standards but also supports your commercial and clinical goals.
Training & Coaching
At SignifyQ our Training & Coaching programs are designed to empower life science professionals with the knowledge, mindset, and confidence to maintain compliance and quality excellence.
Training Domains
Post-Approval Lifecycle Management
Equipping professionals with regulatory frameworks, submission processes, and post-approval compliance requirements across major markets.
Highlights:
Quality Systems & Compliance Training
Comprehensive modules covering GxP fundamentals, deviation management, and inspection readiness — designed for QA, QC, production, and R&D teams.
Programs Include:
Leadership & Compliance Coaching
Customized one-on-one or group coaching sessions for leaders to strengthen compliance culture, communication, and decision-making.
Coaching Focus Areas:
Pharmacovigilance & Safety Monitoring
Training teams to manage global safety systems, signal detection, and case management procedures efficiently.
Covers:

Quality Systems & Compliance Training
Programs Include:
Post-Approval Lifecycle Management
Highlights:


Pharmacovigilance & Safety Monitoring
Covers:

Leadership & Compliance Coaching
Coaching Focus Areas:

Our Training Methodology
Delivery Formats
On-site Workshops
Virtual Interactive Modules
Blended Learning
Certification-Based Programs

Investment Solutions
Need Help? Call Us Now
+234 567 8113Driving Excellence Through Compliance, Control, and Continuous Improvement
We guide our clients through difficult issues, bringing insight and judgment to each situation. Our innovatve approaches create original solutions to our clients’ most complex domestic & multi juristictional deal sid disputes. By thinking on behalf of our clients every day, we anticipate what they want, ineed and build lasting relationships.
Over the last 31 Years we made an impact, we have long way to go.
These are the concepts that shape our distinctive culture & differentiate us from others. They true the unique spirit of our Firm guide the behaviors that enable us to deliver the promises we make to our clients and our people.
Service Process
Over the last 31 Years we made an impact, we have long way to go.
1. How can financial consulting benefit my business?
2. What distinguishes your firm from others in the industry?
3. Do you offer services tailored to specific industries?
4. How can financial consulting benefit my business?
Service Options